

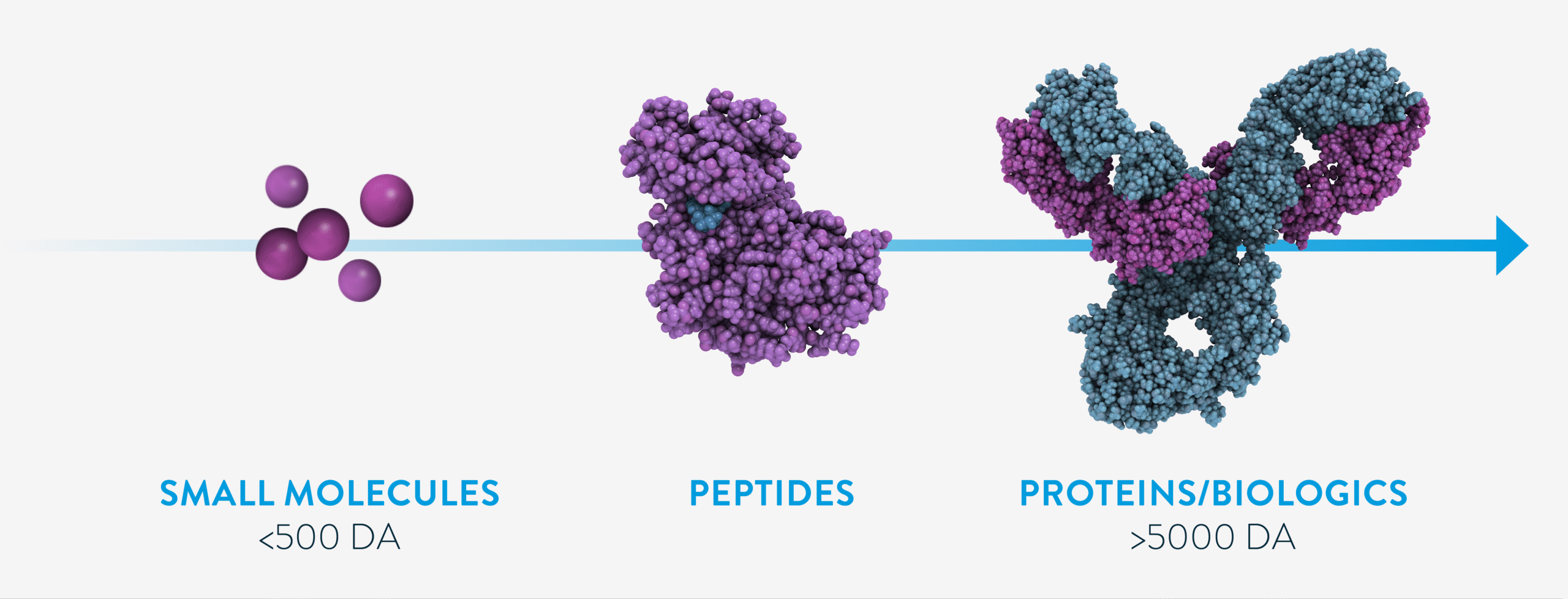
Biologics are complex, large-molecule medicines derived from living cells or produced through biological processes.1 Unlike traditional small-molecule drugs made via chemical synthesis, biologics are developed using biotechnology and manufactured in living cells cultured in vitro.1

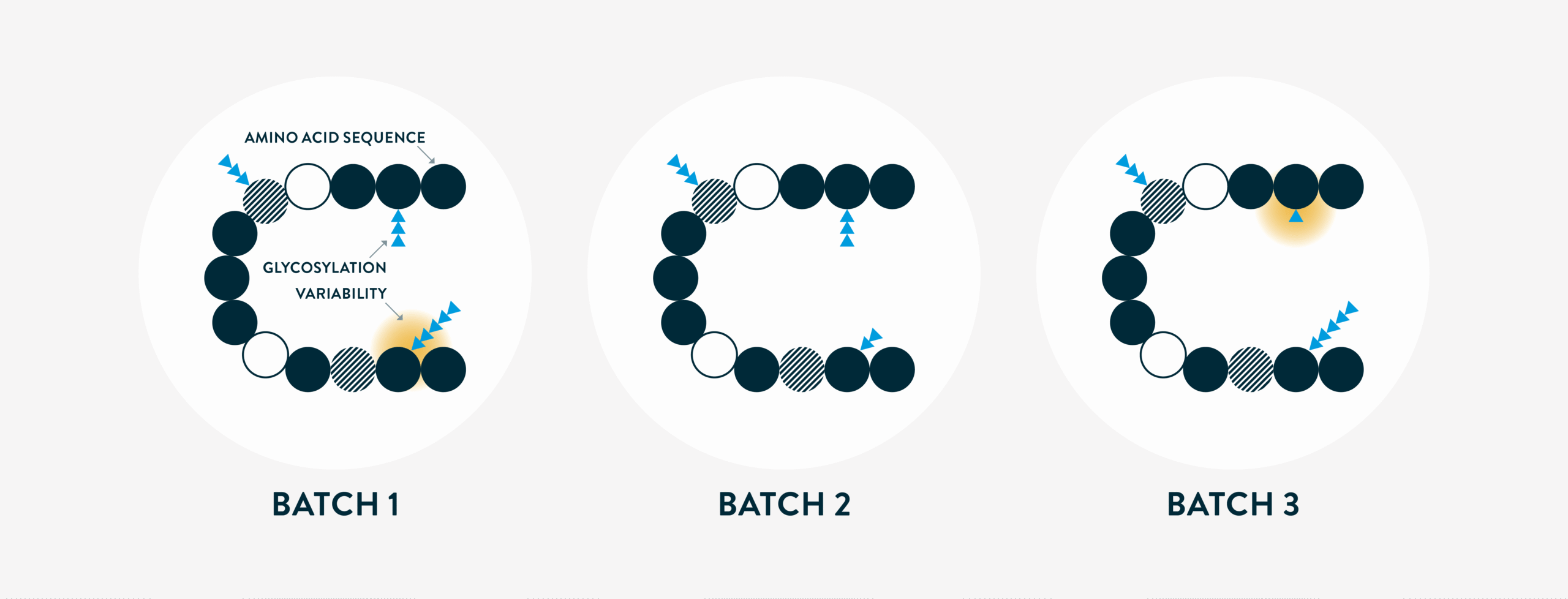
Inherent small degrees of variability can be found within consecutive batches of the same biological medicine; for example, in glycosylation,
where the attached sugar molecules can vary slightly. However, even with minor variations in glycosylation patterns (that fall within acceptable limits), there will still be consistency in biological activity between batches. This means that the biological activity of the medicine (encompassing its safety and effectiveness) is consistent across all batches.
Ultimately, the variations are within an accepted range and do not affect the protein’s function.
Strict controls are always applied to ensure that minor variability falls within an acceptable range to certify safety and efficacy. This is done by altering the manufacturing process to guarantee the active substance aligns with the desired specifications range. Whether variability is within a batch or between batches, these quality assurance measures are always in place.

Biologics for HCPs
What are Biosimilars?
A biosimilar is a biological medicine that is highly similar to an already approved reference medicine in terms of structure, b...

Biologics for HCPs
Extrapolation
If a biosimilar is highly similar to a reference medicine and has comparable safety and efficacy in one therapeutic indication,...

Biologics for HCPs
Articles of Interest
This section serves as a dedicated hub for a curated selection of scientific articles and studies that explore the efficacy and...

Biologics for HCPs
Interchangeability
Interchangeability refers to the exchange of one medicine for another medicine that is expected to have the same clinical effec...

Biologics for HCPs
Biosimilars Benefits
It is estimated that biosimilars will generate savings of up to $290 billion globally by 2027, freeing up healthcare systems to...

Biologics for HCPs
Approval Pathway
While small molecule development typically requires an investment of $2–3 million and takes 2–3 years, developing a biosimilar ...
GLO-25-1335