


Thanks for registering
We encountered a previous register of this Medical ID. An email has been sent for instructions to activate this account.

Welcome to Abbott platform


A code was sent to you via email, please insert the verification number



En nuestra base de datos encontramos un registro previo.
Se ha enviado a tu correo electrónico un email para activar tu cuenta.


No se pudo validar su cuenta.
test.
Biologics for HCPs
January 7, 2026

If a biosimilar is highly similar to a reference medicine and has comparable safety and efficacy in one therapeutic indication, safety and efficacy data may be extrapolated to other indications approved for the reference medicine.1 Both the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) apply this principle, using the totality of evidence to determine whether such extrapolation is scientifically justified.2,3
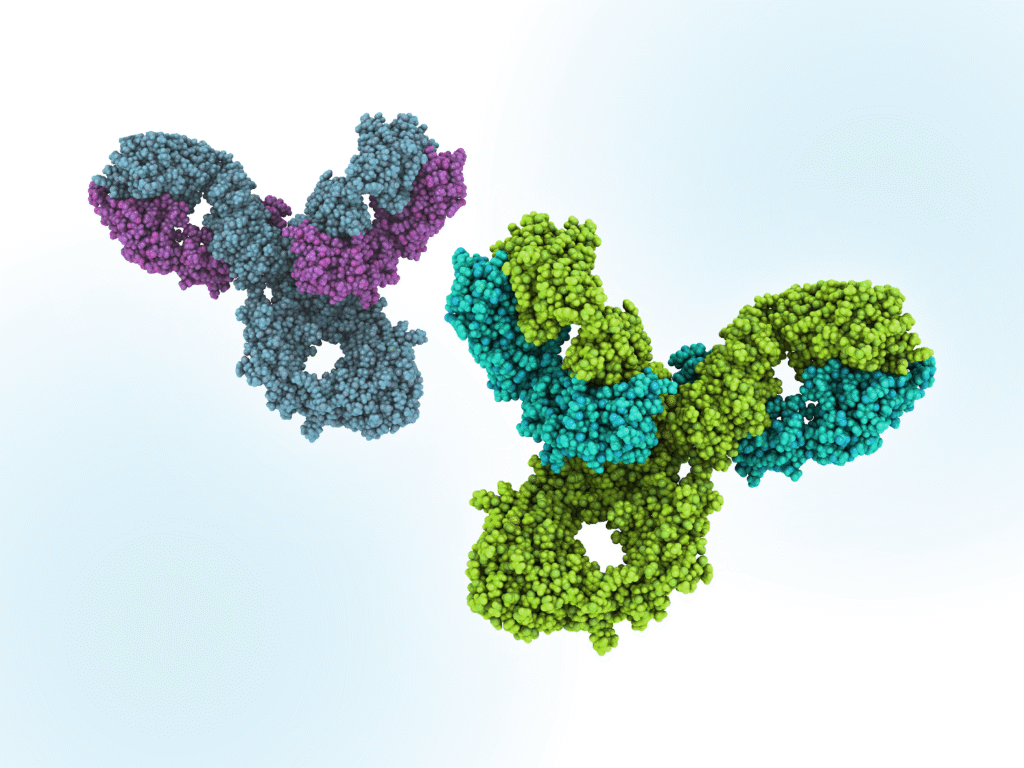
Below are some of the EMA’s specific considerations when assessing extrapolation:1

Mechanism of action:
The active substance must act through the same receptor(s) in both the original & extrapolated indications; if its mechanism involves multiple receptors, additional studies may be required to confirm similar behaviour in the new indication.
Relevant study population:
Comprehensive studies must show the biosimilar is highly similar to the reference medicine in terms of safety, efficacy, and immunogenicity, using a key indication where any potential differences can be detected.

Safety:
Safety data can only be extrapolated once a similar safety profile is confirmed in one indication. If structural, functional, pharmacokinetic/pharmacodynamic, and efficacy comparability is shown, similar adverse reactions and frequencies can be expected.

Immunogenicity: Immunogenicity data cannot be automatically extrapolated, as it depends not only on the product but also on patient, disease, and treatment-related factors, and must always be justified.
GLO-25-1335