


Thanks for registering
We encountered a previous register of this Medical ID. An email has been sent for instructions to activate this account.

Welcome to Abbott platform


A code was sent to you via email, please insert the verification number



En nuestra base de datos encontramos un registro previo.
Se ha enviado a tu correo electrónico un email para activar tu cuenta.


No se pudo validar su cuenta.
test.
Biologics for HCPs
January 7, 2026

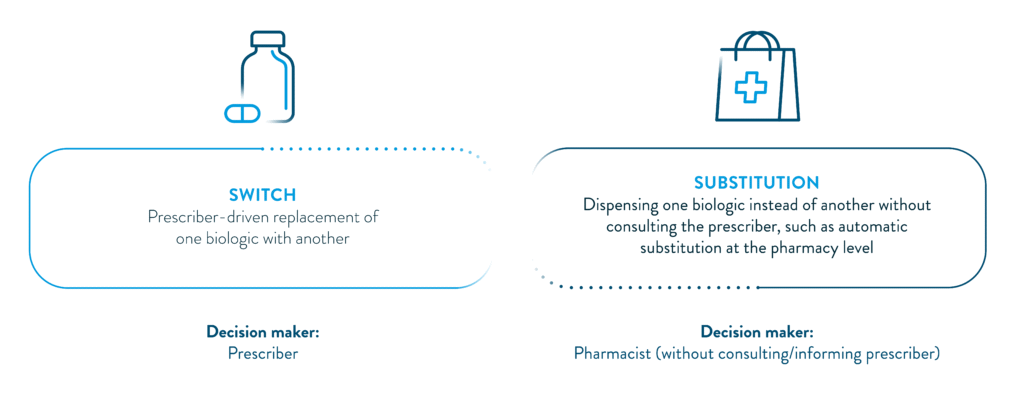
Interchangeability refers to the exchange of one medicine for another medicine that is expected to have the same clinical effect.1 In the context of biosimilars, this means an approved biosimilar can be used instead of its reference product (or vice versa), or one biosimilar can be replaced with another biosimilar of the same reference product.1
REGIONAL DIFFERENCES IN SUBSTITUTION POLICY
While the scientific principles underpinning biosimilar approval are somewhat harmonised on a global scale, how interchangeability is implemented varies widely. This is because substitution policies are shaped not only by regulatory designations, but also by national laws and healthcare system structures within different markets.3 Some regions allow prescribers full control over switching decisions, while others allow pharmacists to substitute at the point of dispensing without consulting the prescriber.2,4,5


The EMA and HMA consider biosimilars interchangeable with their reference product once approved, but switching or substitution should be based on approved use and national policies. Decisions on how interchangeability is applied, by prescribers or at the pharmacy level, are made by individual EU member states, not the EMA.4

An interchangeable biosimilar can be substituted for the reference biologic at the pharmacy without prescriber consultation, similar to generics, subject to state laws. Both biosimilars and interchangeable biosimilars are proven to be as safe and effective as the original biologic and can be prescribed in its place.5
Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration; HMA, Human Medicines Agency.
GLO-25-1335