


Thanks for registering
We encountered a previous register of this Medical ID. An email has been sent for instructions to activate this account.

Welcome to Abbott platform


A code was sent to you via email, please insert the verification number



En nuestra base de datos encontramos un registro previo.
Se ha enviado a tu correo electrónico un email para activar tu cuenta.


No se pudo validar su cuenta.
test.
Biologics for HCPs
January 7, 2026

While small molecule development typically requires an investment of $2–3 million and takes 2–3 years, developing a biosimilar can demand $100–200 million and span 8–10 years.1 A comparative, step-wise approach to biosimilar approval is adopted by regulatory bodies such as the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) to ensure that the drug maintains safety and efficacy to the reference biologic.
The proposed drug must undergo a series of testing, including non-clinical analytical, pharmacological and confirmatory clinical studies to prove biosimilarity to the reference product.2 The critical and non-critical quality attributes are closely matched to the reference biologic via a series of advanced analytical techniques. Each attribute is thoroughly characterised for the originator, and the biosimilar must be reverse engineered with a high degree of molecular comparability.2,3

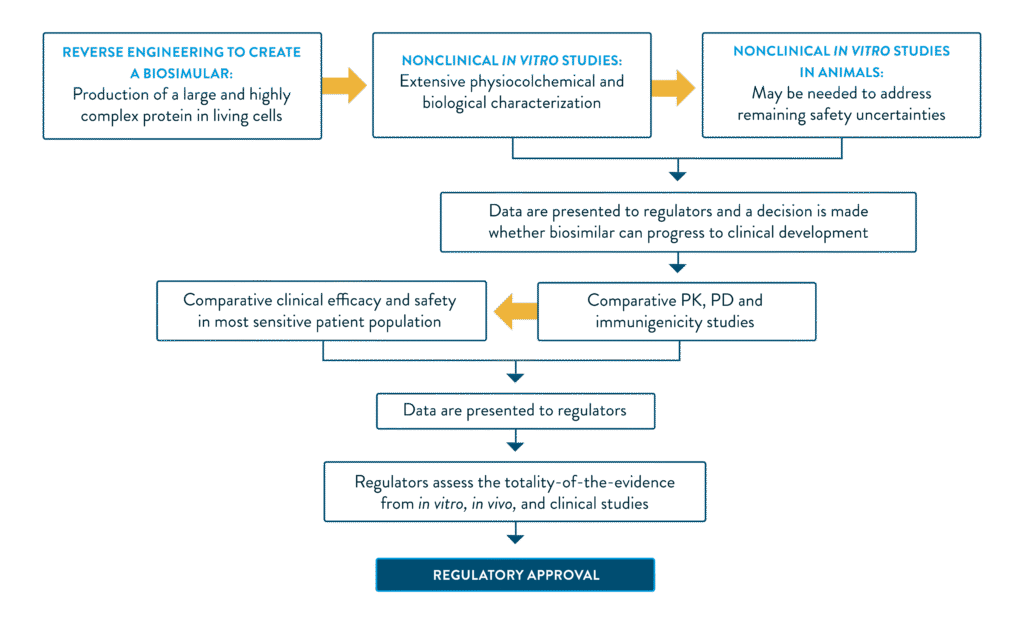
Adapted from Rugo et al. 2016.2
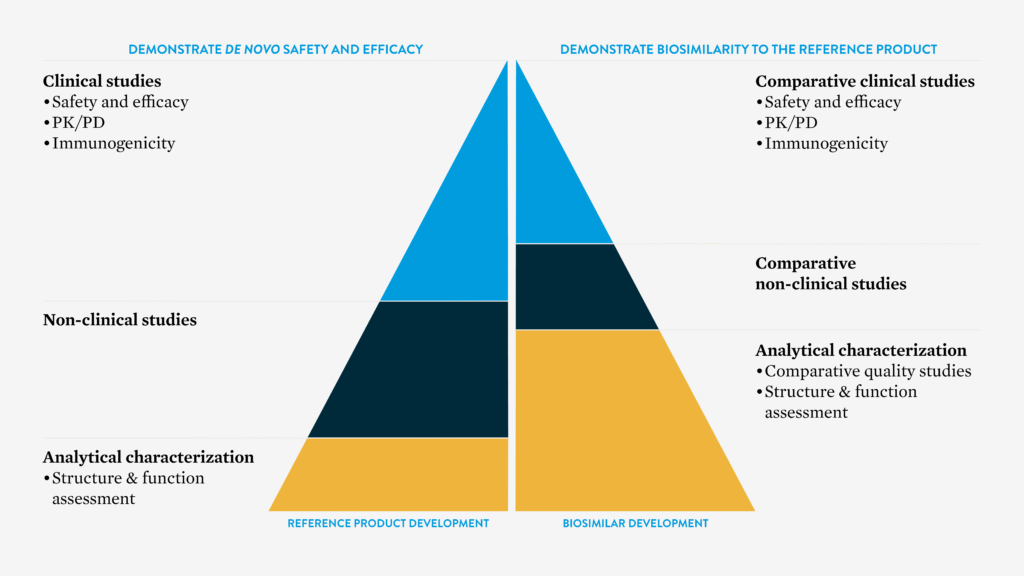
European Medicines Agency4
Once approved, biosimilars can be prescribed in place of their reference products, offering patients proven treatment alternatives that could broaden access, reduce costs, and support more sustainable healthcare systems.
Abbreviations: PD, pharmacodynamic; PK, pharmacokinetic.
GLO-25-1335