What are Biosimilars?
Biologics for HCPs
January 7, 2026

A biosimilar is a biological medicine that is highly similar to an already approved reference medicine in terms of structure, biological activity, efficacy, safety and immunogenicity.1
BIOLOGICS
- Made by or extracted from living organisms, tissues, or cells
- Innovative treatments that have transformed the lives of millions of patients with disabling or life-threatening diseases
- Different batches of the same biological medicine may show a small degree of variability
BIOSIMILAR
- Made by or extracted from living organisms, tissues, or cells
- Matches the reference medicine in terms of safety, efficacy, quality and clinical outcome
- A successor to a ‘reference medicine’ for which the patent has expired and exclusivity has been lost
GENERIC
- Made through chemical synthesis by combining specific chemicals
- A copy of a ‘reference medicine’ for which the patent has expired and exclusivity has been lost
- A small molecule which can be analyzed to determine all its components and shown to be identical to the originator
The term ‘biosimilar’ is recognised and designated by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).2,3 Regulatory bodies require a robust and comprehensive collation of data for a biosimilar to be approved.
This review of comparability focuses on demonstrating that the biosimilar is highly similar to the reference medicine. It includes a step-wise evaluation that begins with extensive comparative analytical and functional studies. Clinical data, typically including a comparative clinical pharmacology study and often a comparative clinical efficacy and safety study, is also required to address any residual uncertainties from earlier analyses.2,3
CONCEPTS OF BIOSIMILARS1,2,4
A table of definitions for some key terms related to biosimilars. A clear understanding of this terminology is essential for navigating the topic of biosimilar medicines.
| Biologic | Biologics are complex, large-molecule medicines derived from living cells or produced through biological processes. |
| Biosimilar | A biological medicine that is highly similar to an already approved reference medicine in terms of structure, biological activity, efficacy, safety and immunogenicity. |
| Extrapolation | Extension of the efficacy and safety data from a therapeutic indication for which the biosimilar has been clinically tested to another therapeutic indication approved for the reference medicine. |
| Generic | A medicine that is developed to be the same as a medicine that has already been authorised. Its authorisation is based on efficacy and safety data from studies on the authorised medicine. A company can only market a generic medicine once the 10-year exclusivity period for the original medicine has expired. |
| Interchangeability | The ability to exchange one medicine for another medicine that is expected to have the same clinical effect. |
| Microheterogeneity | Minor molecular variability among biological substances due to natural biological variability and slight alterations to production methods. |
| Substitution | Practice of dispensing one medicine instead of another equivalent and interchangeable medicine at pharmacy level without consulting the prescriber. |
| Switching | When the prescriber decides to exchange one medicine for another medicine with the same therapeutic intent. |
GLO-25-1335
REFERENCES
- Makurvet FD. Biologics vs small molecules: Drug costs and patient access. Med Drug Discov. 2021;9:100075.
- European Medicines Agency. Biosimilar medicines: overview. Accessed October 2025. Available at: https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview.
- U.S. Food and Drug Administration. Biosimilars: review and approval. Accessed October 2025. Available at: https://www.fda.gov/drugs/biosimilars/review-and-approval.
- European Medicines Agency. Biosimilars in the EU: Information Guide for Healthcare Professionals. Published 2017. Accessed October 2025. Available at: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf
Extrapolation
Biologics for HCPs
January 7, 2026

If a biosimilar is highly similar to a reference medicine and has comparable safety and efficacy in one therapeutic indication, safety and efficacy data may be extrapolated to other indications approved for the reference medicine.1 Both the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) apply this principle, using the totality of evidence to determine whether such extrapolation is scientifically justified.2,3
Below are some of the EMA’s specific considerations when assessing extrapolation:1

Mechanism of action:
The active substance must act through the same receptor(s) in both the original & extrapolated indications; if its mechanism involves multiple receptors, additional studies may be required to confirm similar behaviour in the new indication.
Relevant study population:
Comprehensive studies must show the biosimilar is highly similar to the reference medicine in terms of safety, efficacy, and immunogenicity, using a key indication where any potential differences can be detected.

Safety:
Safety data can only be extrapolated once a similar safety profile is confirmed in one indication. If structural, functional, pharmacokinetic/pharmacodynamic, and efficacy comparability is shown, similar adverse reactions and frequencies can be expected.

Immunogenicity: Immunogenicity data cannot be automatically extrapolated, as it depends not only on the product but also on patient, disease, and treatment-related factors, and must always be justified.
REFERENCES
- European Medicines Agency. Biosimilars in the EU: Information Guide for Healthcare Professionals. Published 2017. Accessed October 2025. Available at: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf.
- European Medicines Agency. Reflection paper on a tailored clinical approach in biosimilar development. Published 2014. Accessed October 2025. Available at: https://www.ema.europa.eu/en/documents/other/reflection-paper-tailored-clinical-approach-biosimilar-development_en.pdf.
- U.S. Food and Drug Administration. Biosimilar Development Process. Published 2017. Accessed October 2025. Available at: https://www.fda.gov/files/drugs/published/Biosimilar-Development-Process.pdf
GLO-25-1335
Interchangeability
Biologics for HCPs
January 7, 2026

Interchangeability refers to the exchange of one medicine for another medicine that is expected to have the same clinical effect.1 In the context of biosimilars, this means an approved biosimilar can be used instead of its reference product (or vice versa), or one biosimilar can be replaced with another biosimilar of the same reference product.1
REGIONAL DIFFERENCES IN SUBSTITUTION POLICY
While the scientific principles underpinning biosimilar approval are somewhat harmonised on a global scale, how interchangeability is implemented varies widely. This is because substitution policies are shaped not only by regulatory designations, but also by national laws and healthcare system structures within different markets.3 Some regions allow prescribers full control over switching decisions, while others allow pharmacists to substitute at the point of dispensing without consulting the prescriber.2,4,5


The EMA and HMA consider biosimilars interchangeable with their reference product once approved, but switching or substitution should be based on approved use and national policies. Decisions on how interchangeability is applied, by prescribers or at the pharmacy level, are made by individual EU member states, not the EMA.4

An interchangeable biosimilar can be substituted for the reference biologic at the pharmacy without prescriber consultation, similar to generics, subject to state laws. Both biosimilars and interchangeable biosimilars are proven to be as safe and effective as the original biologic and can be prescribed in its place.5
REFERENCES
Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration; HMA, Human Medicines Agency.
- European Medicines Agency. Biosimilars in the EU: Information Guide for Healthcare Professionals. Published 2017. Accessed October 2025. Available at: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf.
- Afzali A, et al. The automatic substitution of biosimilars: definitions of interchangeability are not interchangeable. Adv Ther. 2021;38(5):2077–2093.
- Knox RP, et al. Biosimilar approval pathways: comparing the roles of five medicines regulators. J Law Biosci. 2024;11(2):1-27.
- European Medicines Agency. Statement on the scientific rationale supporting the interchangeability of biosimilar medicines in the EU. Published September 19, 2022. Accessed October 2025. Available at: https://www.ema.europa.eu/en/documents/public-statement/statement-scientific-rationale-supporting-interchangeability-biosimilar-medicines-eu_en.pdf.
- U.S. Food and Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Published July 28, 2021. Accessed October 2025. Available at: https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices.
GLO-25-1335
Approval Pathway
Biologics for HCPs
January 7, 2026

While small molecule development typically requires an investment of $2–3 million and takes 2–3 years, developing a biosimilar can demand $100–200 million and span 8–10 years.1 A comparative, step-wise approach to biosimilar approval is adopted by regulatory bodies such as the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) to ensure that the drug maintains safety and efficacy to the reference biologic.
The proposed drug must undergo a series of testing, including non-clinical analytical, pharmacological and confirmatory clinical studies to prove biosimilarity to the reference product.2 The critical and non-critical quality attributes are closely matched to the reference biologic via a series of advanced analytical techniques. Each attribute is thoroughly characterised for the originator, and the biosimilar must be reverse engineered with a high degree of molecular comparability.2,3

THE BIOSIMILAR DEVELOPMENT AND APPROVAL PROCESS
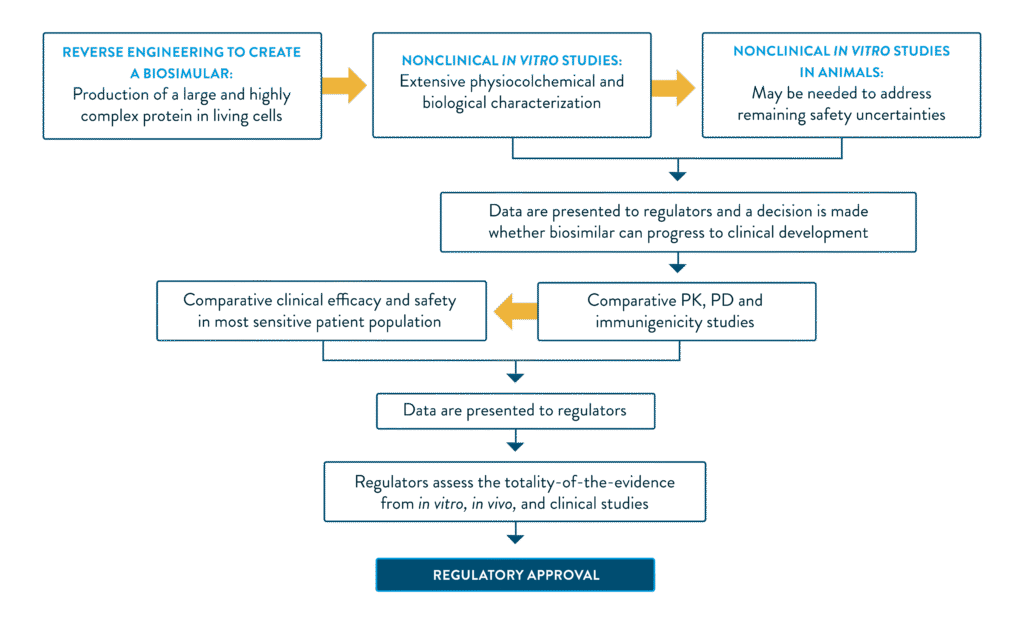
Adapted from Rugo et al. 2016.2
COMPARISON OF DATA REQUIREMENTS FOR APPROVAL OF A BIOSIMILAR VERSUS THE REFERENCE MEDICINE4
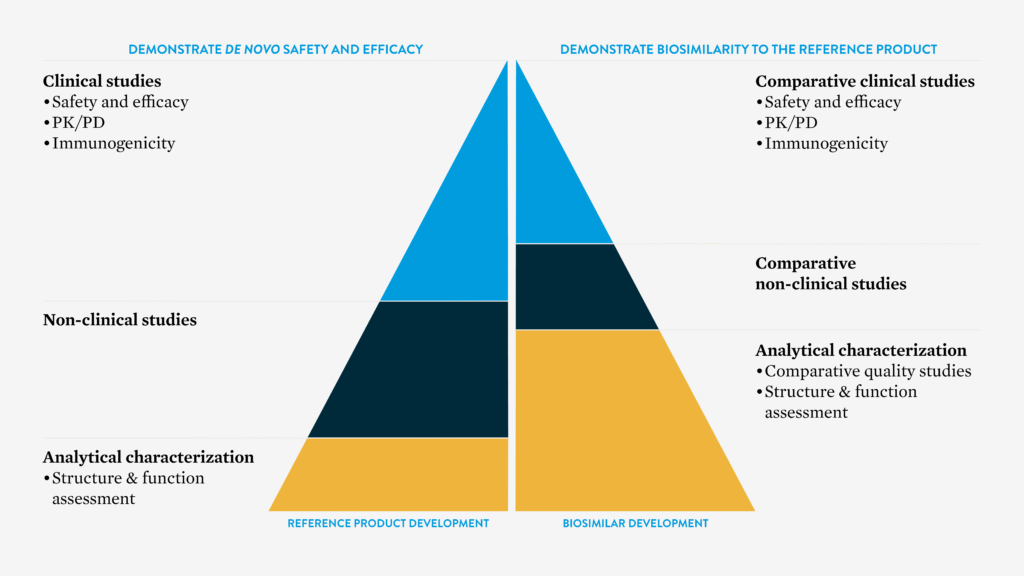
European Medicines Agency4
Once approved, biosimilars can be prescribed in place of their reference products, offering patients proven treatment alternatives that could broaden access, reduce costs, and support more sustainable healthcare systems.
REFERENCES
Abbreviations: PD, pharmacodynamic; PK, pharmacokinetic.
- GaBI Online. Comparison of the cost of development of biologicals and biosimilars. Generics and Biosimilars Initiative Journal. Published March 11, 2022. Accessed October 2025. Available at: https://gabionline.net/reports/comparison-of-the-cost-of-development-of-biologicals-and-biosimilars.
- Rugo HS, Rifkin RM, Declerck P, Bair AH, Morgan G, Woollett G, et al. A clinician’s guide to biosimilars in oncology. Cancer Treat Rev. 2016;46:73–9. Accessed October 2025. Available at: https://www.sciencedirect.com/science/article/pii/S0305737216300172.
- Nupur N, Singh SK, Rathore AS. Analytical similarity assessment of biosimilars: global regulatory landscape, recent studies and major advancements in orthogonal platforms. Front Bioeng Biotechnol. 2022;10:832059.
- European Medicines Agency. Biosimilars in the EU: Information Guide for Healthcare Professionals. Published 2017. Accessed October 2025. Available at: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf.
GLO-25-1335
Biosimilars Benefits
Biologics for HCPs
January 7, 2026

It is estimated that biosimilars will generate savings of up to $290 billion globally by 2027, freeing up healthcare systems to support more people.1 They are also improving affordability.2 For instance, where an originator biologic medicine can require an $800 million investment and 8–10 years of development, biosimilars can markedly reduce this investment by as much as eightfold.2 These scientifically developed alternatives to originator biologics can offer substantial cost savings that could be reinvested into healthcare systems, unlocking broader access to life-changing therapies.
PRICE DIFFERENCES PER COUNTRY BETWEEN BIOSIMILARS AND REFERENCE BIOLOGIC.3
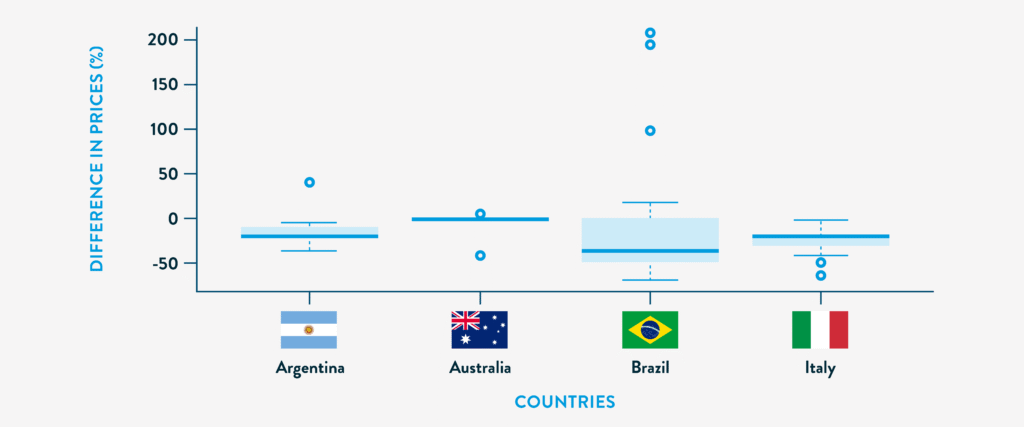
In a cross-national study of biosimilar pricing across Argentina, Brazil, Italy, and Australia; Brazil showed the highest median price reduction at 36.3%, with nearly half of its biosimilars priced over 40% below the originator.3
With biosimilars, physicians have more treatment options to suit diverse patient needs, while patients – especially those in resources constrained environments – can gain greater access to biologic medicines. This is especially important in middle to low-income countries, where high costs have traditionally limited the use of biologics.4 Biosimilars can help close this gap, facilitating more equitable healthcare delivery on a global scale.

GLOBAL OVERVIEW OF AVAILABILITY OF GENERAL AND INSULIN-SPECIFIC BIOSIMILAR GUIDELINES.5
However, the global picture is still uneven. In some countries, biosimilar regulatory guidelines have yet to be established, or their status remains unknown, which could slow patient access.5 Addressing these gaps is essential to ensuring that the benefits of biosimilars reach patients everywhere, not just in markets with established frameworks.

FUELLING SUSTAINABLE HEALTHCARE
In 2021, biologics made up nearly half of all U.S. prescription medicine spending, totalling $256 billion, despite representing only around 3% of prescriptions.6 In 2024, the European Union spent €95 billion on biologics, accounting for 41% of its pharmaceutical expenditures.7 This level of spending is not sustainable for patients or health systems.
Biosimilars play a critical role in addressing this imbalance. By fostering constructive market competition, they help lower costs and expand access to life-changing treatments.7 In fact, as of July 2024, the cumulative savings at list prices from the impact of biosimilar competition in Europe reached €56 billion.7
As more biosimilars reach the market (with 40 FDA-approved and over 100 in development*) they offer a scalable solution to rising healthcare costs. When biosimilars are adopted, both biosimilars and their reference biologics become more affordable, freeing up resources that can be reinvested in other areas of care.8
REFERENCE
As of 2023.
- IQVIA. The Global Use of Medicines 2023. Accessed October 2025. Available at: https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicines-2023.
- GaBI Online. Comparison of the cost of development of biologicals and biosimilars. Generics and Biosimilars Initiative Journal. Published March 11, 2022. Accessed October 2025. Available at: https://gabionline.net/reports/comparison-of-the-cost-of-development-of-biologicals-and-biosimilars.
- Machado FLS, Cañás M, Urtasun MA, Marín GH, Albuquerque FC, Pont L, et al. A cross-national comparison of biosimilars pricing in Argentina, Australia, Brazil, and Italy. Ther Innov Regul Sci. 2024;58:549–56.
- World Health Organization. Biosimilars: Expanding Access to Essential Biologic Therapies. Published February 13, 2025. Accessed October 2025. Available at: https://www.who.int/news/item/13-02-2025-biosimilars–expanding-access-to-essential-biologic-therapies.
- Heinemann L, Khatami H, McKinnon R, Home PD. An overview of current regulatory requirements for approval of biosimilar insulins. Diabetes Technol Ther. 2015;17(7):510–6.
- U.S. Food and Drug Administration. FDA and FTC Collaborate to Advance Competition in the Biologic Marketplace. Published March 2, 2023. Accessed October 2025. Available at: https://www.fda.gov/news-events/fda-voices/fda-and-ftc-collaborate-advance-competition-biologic-marketplace.
- IQVIA. The Impact of Biosimilar Competition in Europe 2024. Accessed October 2025. Available at: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2024.pdf.
- Kvien TK, Patel K, Strand V. The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum. 2022;52:151941.
GLO-25-1335
Articles of Interest
Biologics for HCPs
January 7, 2026

This section serves as a dedicated hub for a curated selection of scientific articles and studies that explore the efficacy and real-world effectiveness of biosimilars.
Here, you will find links to foundational research and real-world evidence demonstrating how biosimilars expand treatment choices for patients. Explore the articles linked below to delve deeper into the science behind biosimilars.
What Are Biosimilars, and How Can They Help You? | Abbott Newsroom
What are Biologic Medicines and Biosimilars? | Abbott Newsroom
Our biosimilars in women’s health – Abbott | Medicines GLOBAL
Our biosimilars in oncology – Abbott | Medicines GLOBAL
GLO-25-1335








